it isnt natual in there system apparently the episode they had to stop a giant asteroid sent to earth by the gould carter finds there is naquaduh in the asteroid and says it isnt natual to their solar system
Announcement
Collapse
No announcement yet.
No naquada on Earth.
Collapse
X
-
Well the Ancients would have used most of it up as nearly all there tech uses the stuff and they were on Earth for millions of years. Then the Goa'uld were here for a while and they enjoyed a little slave driven naquadah mining every now and again. So if there is any left, it's bound to be a pitiful amount.
Comment
-
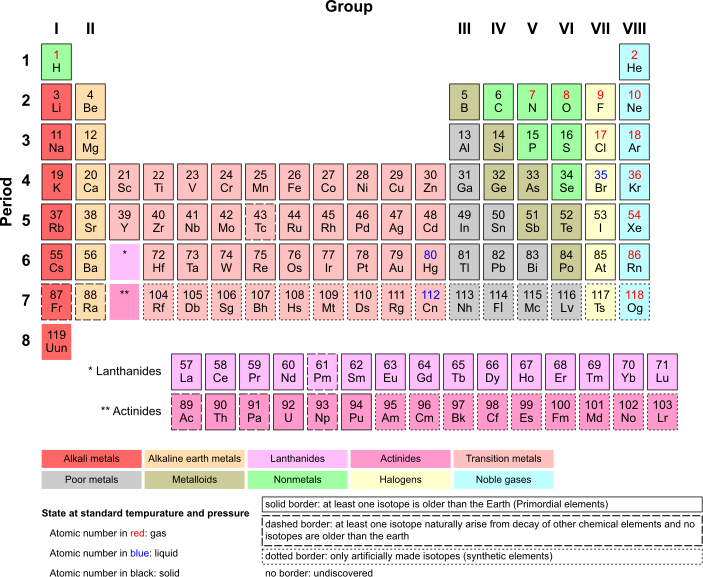
Below is a current periodic table, which contains all elements from 1 to 118, save 117 whose existence has not yet been confirmed. There are only two elements lighter than Uranium that were not found naturally on Earth: Technicium (43) and Promethium (61), both which are difficult to find in nature due to their short half-lives.Originally posted by Aer'ki View PostAs for what naquada is, they have said it is very heavy. I would imagine that it is one of the missing elements from the periodic table. If any of you had noticed, they mislabeled the numbers. There are many element possibilities on the left half of the table that are skipped over because we don't have them on Earth(that we've found). Naquada might be one of these.
Spoiler:
Note that the "hole" between Barium (53) and Hafnium (72) is the lanthanoid series, the elements of which are shown below the table, and the hole between Radium (88) and Rutherfordium (104) is the actinoid series, which is also shown below the table.
However, there is no Naquada anywhere in the solar system. That was an important plot point in "Failsafe": it's how they knew that the asteroid was actually a Goa'uld attack.Originally posted by Mclean View PostWell the Ancients would have used most of it up as nearly all there tech uses the stuff and they were on Earth for millions of years. Then the Goa'uld were here for a while and they enjoyed a little slave driven naquadah mining every now and again. So if there is any left, it's bound to be a pitiful amount."From East Middle School. Suzumiya Haruhi. I have no interest in ordinary humans. If there are any aliens, time travelers, sliders, or espers here, come join me."
- The Melancholy of Haruhi Suzumiya; Best Character Introduction Ever.
"And can we lose the ten thousand year old dead plants?!"
- Stargate: Atlantis (1x03) "Hide and Seek"
"Hammerheads do not load/unload units immediately – they must descend to ground level first. Initial experiments involving jump-jetting infantry into the Hammerhead’s cargo compartment met with unfortunate results."
- Command&Conquer 3: Kane's Wrath Hammerhead Unit Spotlight
Comment
-
I'm not saying that there is a bias one way or another. And I'm not saying that it is a fact confirmed in one way or another. However, it is a very reasonable extrapolation of what we know. Most abundant elements in the Universe are Hydrogen and Helium all others lag behind by a great margin. We know that any rocky worlds form from remains of old stars. Ergo, there is a very good chance that a lot of systems out there lack heavy elements, and therefore, cannot consist of anything other than gas giants.Originally posted by Aer'ki View PostWe don't know that for a fact. A few decades ago we didn't know that there were planets outside our own star system. Right now, we can only find the really big planets in other star systems because of the gravitational tug they put on the stars.
There may very well be small rocky worlds in the Proxima System, the closest star system to us, but because they don't have enough pull to tug on the star enough for us to measure, we're not going to detect them.
So don't assume that there is a statistical bias toward light element planets out there.
Also, there is a great number of signs used to detect extra-solar planets. Planets as light as 5-11 times the mass of Earth have been detected. Exoplanets.
Comment
-
i read something about there being a chance of finding Star Trek - like planets, ie planets made entirely of 1 thing, like only ice, only water, only a form of rock.
also, a gas giant is a pretty simple form of planet. i think a gas giant forms much easier than a rock planet.
but we dont know enough yet. small planets are still hard to detect
Comment
-
That's a theory. We don't have any evidence to support that. It's just a postulation to tie up a lot of loose ends. It's also ironic that scientists so easily accept the unanswered existence of hydrogen, but must have a reason for the heavier elements to exist.Originally posted by K^2 View PostI'm not saying that there is a bias one way or another. And I'm not saying that it is a fact confirmed in one way or another. However, it is a very reasonable extrapolation of what we know. Most abundant elements in the Universe are Hydrogen and Helium all others lag behind by a great margin. We know that any rocky worlds form from remains of old stars. Ergo, there is a very good chance that a lot of systems out there lack heavy elements, and therefore, cannot consist of anything other than gas giants.
Also, there is a great number of signs used to detect extra-solar planets. Planets as light as 5-11 times the mass of Earth have been detected. Exoplanets.
One unavoidable fact about scientists is that they don't like having to say "We don't know" so they come up with all kinds of 'theories' to the plug the holes.
And if we are only seeing planets 5 times larger than Earth, consider how many of that size there are in our system. Anything Earth size isn't seen. I'd call that a big detection gap.
Comment
-
No, I wasn't. I meant the shell gaps in the progression of the elements. The Periodic Table is numbered based on the number of protons, but if you look at the electron progression in the shells you will notice that it skips over some gaps.Originally posted by Splitsecond View PostThere are no missing elements in the periodic table up to the latest confirmed element, which will probably be called copericium, which is element 112. The gap that I think you are referring to is due to the rare earth metals group normally being set aside from the main group of elements for classification reasons.
For example...Nickel and Copper. Copper is one Proton up on Nickel, which means one more electron to balance it out. But, Nickel is +2, and Copper is +1. As you go left to right in the periodic table you pick up electrons in the outer shell, but not between these two. An electron is taken out of the outer shell and deposited in an inner shell. You are going from 16/2 to 18/1...leaving out 17/2. 18/2 is Zinc.
So even though all the protons are accounted for, what would a 17/2 configuration behave as? This is what I mean by missing elements in the table.
Comment
-
Aer'ki, that's simply the order in which the shells fill out. There are no gaps. The 4s orbital fills out before the 3d, because 4s states have lower energy than the 3d states. If you had actually studied some physics, you'd know why, and be able to derive the actual energy levels. Next best thing is having a look at this article.
Edit: Protons and neutrons are the first thing that will condense as a cloud of quark gluon plasma expands. Free neutrons decay into protons and electrons. So primordial ionized gas will be primarily hydrogen. I'm not getting into it deeper without you first learning a few things about time.
Comment
-
You missed my point. The 4s was already full and the 3d was filling in sequence, then it broke sequence. It pulled back an electron from the 4s. There is no element with a 3d9. It skips it.Originally posted by K^2 View PostAer'ki, that's simply the order in which the shells fill out. There are no gaps. The 4s orbital fills out before the 3d, because 4s states have lower energy than the 3d states. If you had actually studied some physics, you'd know why, and be able to derive the actual energy levels. Next best thing is having a look at this article.
Edit: Protons and neutrons are the first thing that will condense as a cloud of quark gluon plasma expands. Free neutrons decay into protons and electrons. So primordial ionized gas will be primarily hydrogen. I'm not getting into it deeper without you first learning a few things about time.
Read though what I say next time a bit more thorough before you begin the patronization.
Comment
-
If that is your answer to anything beyond "what is that?", then you have no business in the science sections of the board.Originally posted by Aer'ki View PostThat's a theory.
Then it is not a theory. Theories, by their very definition, have to have strong evidential support.Originally posted by Aer'ki View PostWe don't have any evidence to support that.
Yeah: that's because hydrogen is simple. It's one proton with an electron bonded to it. If you are going to have anything heavier than Hydrogen, then you have to start bonding protons together, which is far more difficult than attaching an electron to a proton.Originally posted by Aer'ki View PostIt's also ironic that scientists so easily accept the unanswered existence of hydrogen, but must have a reason for the heavier elements to exist.
Those gaps are there due to the way that electrons behave inside the atoms: different electrons inside an atom are located at different energy levels, and thus it is far easier to eject some electrons than it is others.Originally posted by Aer'ki View PostNo, I wasn't. I meant the shell gaps in the progression of the elements. The Periodic Table is numbered based on the number of protons, but if you look at the electron progression in the shells you will notice that it skips over some gaps.
For example...Nickel and Copper. Copper is one Proton up on Nickel, which means one more electron to balance it out. But, Nickel is +2, and Copper is +1. As you go left to right in the periodic table you pick up electrons in the outer shell, but not between these two. An electron is taken out of the outer shell and deposited in an inner shell. You are going from 16/2 to 18/1...leaving out 17/2. 18/2 is Zinc.
For example, in an atom with 29 protons (i.e., copper), there is a broad gap between the electron that is easiest to remove and the next electron after that. You could, with an extraordinary amount of effort, draw a second electron out of a +1 copper ion, but it will not happen naturally because it is so much easier to ionize a second copper ion.
The same is not true of an atom with 28 protons: the first two electrons fall into the same general energy level, and thus it is fairly easy to remove both of them.
It wouldn't, because it doesn't exist.Originally posted by Aer'ki View PostSo even though all the protons are accounted for, what would a 17/2 configuration behave as?"From East Middle School. Suzumiya Haruhi. I have no interest in ordinary humans. If there are any aliens, time travelers, sliders, or espers here, come join me."
- The Melancholy of Haruhi Suzumiya; Best Character Introduction Ever.
"And can we lose the ten thousand year old dead plants?!"
- Stargate: Atlantis (1x03) "Hide and Seek"
"Hammerheads do not load/unload units immediately – they must descend to ground level first. Initial experiments involving jump-jetting infantry into the Hammerhead’s cargo compartment met with unfortunate results."
- Command&Conquer 3: Kane's Wrath Hammerhead Unit Spotlight
Comment
-
You've avoided my point. Why doesn't Copper add the electron to the 3d level and maintain the two outer electrons of Nickel? By sequence that is what it should do, but it doesn't. There is a gap in the electron placement progression.Originally posted by Quadhelix View PostIf that is your answer to anything beyond "what is that?", then you have no business in the science sections of the board.
Then it is not a theory. Theories, by their very definition, have to have strong evidential support.
Yeah: that's because hydrogen is simple. It's one proton with an electron bonded to it. If you are going to have anything heavier than Hydrogen, then you have to start bonding protons together, which is far more difficult than attaching an electron to a proton.
Those gaps are there due to the way that electrons behave inside the atoms: different electrons inside an atom are located at different energy levels, and thus it is far easier to eject some electrons than it is others.
For example, in an atom with 29 protons (i.e., copper), there is a broad gap between the electron that is easiest to remove and the next electron after that. You could, with an extraordinary amount of effort, draw a second electron out of a +1 copper ion, but it will not happen naturally because it is so much easier to ionize a second copper ion.
The same is not true of an atom with 28 protons: the first two electrons fall into the same general energy level, and thus it is fairly easy to remove both of them.
It wouldn't, because it doesn't exist.
And just because it hasn't been observed doesn't mean it doesn't exist. We live on one tiny planet in a vast universe. It is possible that the configuration does exist, along with others where there are 'gaps' in the sequence. Paladium has 0 outer shell electrons where it should have 2, unless the chart I'm looking at is flawed. That shouldn't happen, but it does.
So, to get back to the point, Naquada might be another form of a current element that behaves differently...and a different electron shell configuration could affect the afforementioned superconductivity question.
Comment



Comment